Abstract
Background B cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) are tumor necrosis factor superfamily (TNFSF) members that bind transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI), B-cell maturation antigen, and/or BAFF receptor (BAFF-R) on B cells and together support B-cell development, survival, and differentiation into antibody-secreting cells (ASC). In preclinical studies, co-neutralization of BAFF and APRIL can directly suppress ASC and reduce circulating immunoglobulins. Autoimmune hemolytic anemia (AIHA) and immune thrombocytopenic purpura (ITP) are diseases characterized by autoantibodies directed against red blood cells and platelets, respectively. Significantly higher levels of both BAFF and APRIL have been observed in the serum of patients with AIHA and ITP compared to healthy subjects, and polymorphisms in BAFF and TACI have been associated with ITP [Abdel-Hamid, et al., Am J Med, 2011; Emmerich, et al., Br J Haematol, 2007; Peng, et al., J Thromb Haemost, 2017; Yu, et al., Blood Adv, 2021]. Currently, there are no B cell targeting agents approved to treat AIHA or ITP. Rituximab, an anti-CD20 therapeutic which depletes CD20+ B cells, and thereby only indirectly reduces autoantibody production, has demonstrated some efficacy in both ITP [Neunert, et al., Blood, 2019] and AIHA [Jäger, et al., Blood Rev, 2019], though it is not approved for use in either indication. Belimumab, an anti-BAFF neutralizing antibody (Ab), has demonstrated encouraging efficacy in the treatment of ITP associated with systemic lupus erythematosus (SLE) [De Marchi, et al., Clin Exp Rheumatol, 2017] and also in combination with rituximab in patients with ITP [Mahévas, et al., Haematologica, 2021], but only had a modest impact on hematological manifestations in SLE patients [Manzi, et al., Ann Rheum Dis, 2012]. To address the shortcomings of existing B-cell-targeting agents, we have developed ALPN-303, an Fc fusion of an engineered TACI variant TNFRSF domain, which substantially improves upon the ligand affinity liabilities of wild type (WT) TACI, resulting in highly potent dual BAFF/APRIL inhibition superior to WT TACI-Fc, or to BAFF- or APRIL-specific monoclonal Abs. In preclinical studies, ALPN-303 demonstrates enhanced pharmacokinetic (PK) and immunomodulatory properties vs. WT TACI-Fc, which may translate to lower and/or less frequent doses in humans. ALPN-303 also suppresses autoantibodies and nephritis in mouse models of lupus. Taken together, these observations suggest that a potent dual BAFF/APRIL antagonist such as ALPN-303, by lowering pathogenic autoantibody levels, may be particularly beneficial in the treatment of antibody-related hematologic diseases, such as autoimmune cytopenias.
Methods In this first-in-human study (NCT05034484), healthy adult volunteers have been enrolled in single ascending dose cohorts of intravenously (IV) or subcutaneously (SC) administered ALPN-303. For each IV cohort, the first 2 sentinel subjects are randomized 1:1 to receive ALPN-303 or placebo, followed by the remaining 4 subjects randomized 3:1 to receive ALPN-303 or placebo. SC cohorts are randomized 4:2 to receive a single SC dose of ALPN-303 or placebo. All subjects are followed to assess safety, PK, and pharmacodynamic (PD) effects including impacts on circulating immunoglobulins (Ig), and peripheral B-cell populations.
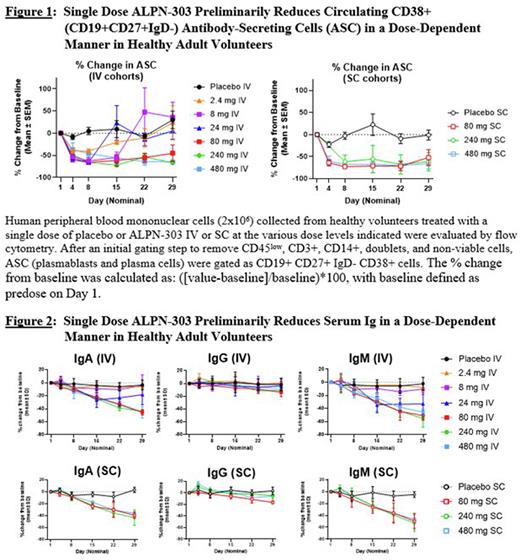
Results ALPN-303 has been well tolerated in all cohorts evaluated to date and exhibits dose-dependent PK and expected PD effects, including dose-dependent reductions in circulating CD38+ (CD19+CD27+IgD-) ASC (Figure 1) and in serum Ig (Figure 2). To date, there have been no serious adverse events, and no events of cytokine release syndrome. Dose escalation will be complete by the time of the meeting; the presentation will include all available safety, PK, and PD data.
Conclusions In this phase 1 study, ALPN-303 demonstrates acceptable preliminary safety and tolerability and exhibits dose-dependent PK and expected PD effects on circulating Ig and B-cell populations. These findings support future clinical development of ALPN-303 in patients with antibody-related diseases, including autoimmune cytopenias.
Disclosures
Dillon:Alpine Immune Sciences: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: co-inventor on several patent applications related to ALPN-303. Davies:Alpine Immune Sciences: Current Employment, Current equity holder in publicly-traded company; Zymeworks: Current equity holder in publicly-traded company, Ended employment in the past 24 months, Patents & Royalties: patent for ZW49. Lickliter:Alpine Immune Sciences: Research Funding; Amplia Therapeutics: Consultancy. Manjarrez:Alpine Immune Sciences: Current Employment. Smith:Alpine Immune Sciences: Current Employment, Current equity holder in publicly-traded company; Pharmacyclics and Abbvie Company: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Lessig:Alpine Immune Sciences: Current Employment, Current equity holder in publicly-traded company; Neoleukin Therapeutics: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Blanchfield:Alpine Immune Sciences: Current Employment, Current holder of stock options in a privately-held company. Sanderson:Alpine Immune Sciences: Current Employment. Chunyk:Alpine Immune Sciences: Current Employment, Current equity holder in publicly-traded company; Aptevo Therapeutics: Ended employment in the past 24 months. Blair:Alpine Immune Sciences: Current Employment. Enstrom:Alpine Immune Sciences: Current Employment, Current equity holder in publicly-traded company; Actym Therapeutics: Ended employment in the past 24 months; Tempest Therapeutics: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Zayed:Alpine Immune Sciences: Current Employment, Current equity holder in publicly-traded company. Peng:Alpine Immune Sciences: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal